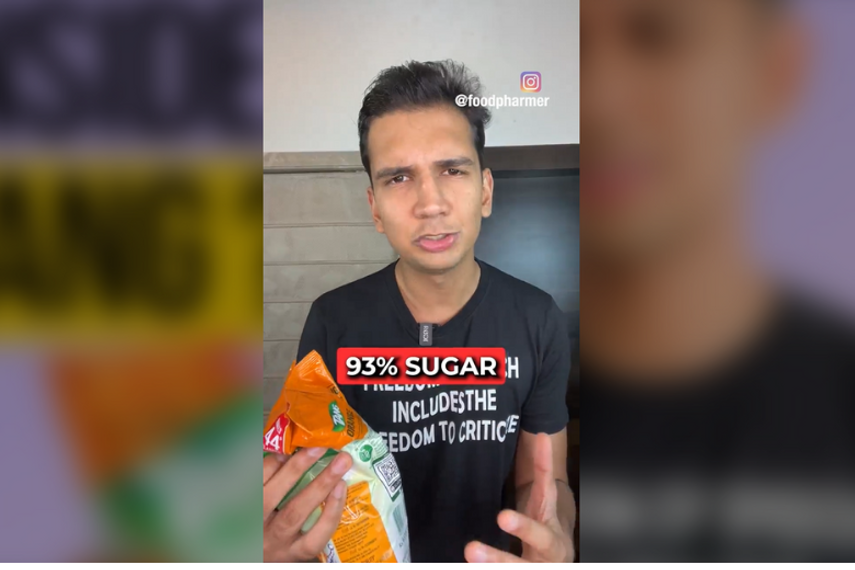
Influencer Revant Himatsingka who earlier took on Mondelez’s Bournvita is at it once again. This time the ‘FoodPharmer'- as he’s better known on social media- has locked horns with another big brand from the company’s kit- the powdered beverage brand, Tang. The health influencer, who has a following of over 600K on just Instagram, has posted a scathing review of the orange drink, proclaiming it contains as much as 93% sugar.
“Tang has ~93% sugar AND contains artificial sweeteners on top!” says Himatsingka in the 90-second video, adding that the popular drink is nothing but “flavoured sugar powder”.
If they limited their marketing to taste, there would be no issue, he points out. The problem is they advertise their product with several “health claims”- as a drink with various vitamins, he says.
Himatsingka cites the example of Tang’s marketing campaign that advertises on TV with the slogan, "Tang Milao, Pani pilao" to get children to drink more water using the flavoured powder.
The influencer goes on to add that Tang also contains E171 (Titanium Dioxide), an ingredient banned by the European Union and several Middle-Eastern countries. While the quantity of Titanium Dioxide in food products is too low to be unsafe for humans according to certain studies, some data suggests that titanium dioxide accumulates in our body over time, he notes.
He also added the disclaimer which said that this product review is made for educational purposes and there is "no intention to defame any company."
In his post Himatsingka also states that he had uploaded a video on Tang in April. But after the legal notice on Bournvita, he removed the video (Mondelez owns both Bournvita and Tang). He later decided to update the Tang video and post it again.
The 31-year old’s bio on LinkedIn says that he has 3 main goals for the next few years, top of which is to stop FMCGs from marketing junk food as healthy food and to teach Indians how to read food labels.
Campaign India reached out to Mondelez for their response to Himatsingka's post on Tang's misleading claims. We are awaiting their reply on this developing story and will update it accordingly.


.jpg&h=334&w=500&q=100&v=20250320&c=1)
.jpg&h=334&w=500&q=100&v=20250320&c=1)


.jpg&h=334&w=500&q=100&v=20250320&c=1)


.jpg&h=334&w=500&q=100&v=20250320&c=1)

.jpg&h=334&w=500&q=100&v=20250320&c=1)








